Seaman, Tami
Page Navigation
-
Watch out for Lead in the Water!
Posted by TAMI SEAMAN on 4/14/2016Lead (Pb), atomic number 82, can cause detrimental health effects in humans. Once ingested lead competes with calcium to be absorbed by the body. The lead then sticks to the red blood cells and them moves into the soft tissues of the body. It is also absorbed by the bones. When lead is absorbed by brain tissue it affects the frontal cortex of the brain, the area responsible for planning, attention, and abstract thought. In addition, it works on the hippocampus the area of the brain that is essential for learning and memory. The strongest effects of lead poisoning are in younger children, especially boys.
What can you do?
1. Have your water tested for Lead.
2. Get your child screened for lead poisoning.
-
Holy Mole-y What a Big Number!
Posted by TAMI SEAMAN on 3/21/2016Holy Mole-y!
This week our class has been celebrating Mole Week.
What is a mole and what does it have to do with Chemistry?

A chemsitry mole has nothing to do with this small burrowing mammal.

A chemistry mole has nothing to do with the mole on your face.
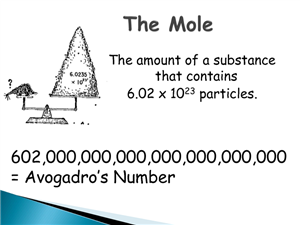
"In chemistry the mole is the amount of a substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12; its symbol is "mol"." http://www.chemistryexplained.com
-
Nikola Tesla was granted patent for “electric magnetic motor” on May 1,1888
Posted by TAMI SEAMAN on 5/1/2015Nikola Tesla was granted a patent for an “electric magnetic motor” on May 1, 1888
Not only was this the first alternating current (AC) motor, but it also heralded a new system of power transmission, which is the basis for the mains power we use today. The Serbian-American physicist, engineer and inventor is recognized as one of the pioneers of electric power. -
April 30th, 1897 Discovery of Electrons by Thompson
Posted by TAMI SEAMAN on 4/28/2015
April 30th
Sir Joseph J. Thomson announced the discovery of the electron on this day in 1897Thomson was studying the properties of cathode rays, and found that they were over 1,000 times lighter than the hydrogen (H) atom and that they were the same mass irrespective of the parent atom. He called these particles “corpuscules”, but scientists later dubbed them “electrons”. -
Chemistry Behind Pop Rocks Candy
Posted by TAMI SEAMAN on 4/27/2015So what’s the science behind Pop Rocks?
Here's the basic idea. Hard candy is made from sugar, corn syrup, water and flavoring. You heat the ingredients together and boil the mixture to drive off all of the water. Then you let the temperature rise. What you are left with is a pure sugar syrup at about 300 degrees F (150 degrees C). When it cools, you have hard candy.
To make Pop Rocks, the hot sugar mixture is allowed to mix with carbon dioxide gas at about 600 pounds per square inch (psi). The carbon dioxide gas forms tiny, 600-psi bubbles in the candy. Once it cools, you release the pressure and the candy shatters, but the pieces still contain the high-pressure bubbles (look at a piece with a magnifying glass to see the bubbles).
When you put the candy in your mouth, it melts (just like hard candy) and releases the bubbles with a loud POP! What you are hearing and feeling is the 600-psi carbon dioxide gas being released from each bubble.
Pop Rocks are back on the shelves today, after a brief re-naming and re-marketing campaign in the 80’s. If you happen to find any in the trick-or-treat pile, and you’re the type to throw caution to the wind… go ahead and down a packet or two with a soda chaser and see what truth there is to the rumors. Please understand however I’m not encouraging this. If you have to find out for yourself, do so at your own risk. I don’t want to be responsible for any exploding science enthusiasts, or even any bad stomach aches.
-
Let's Celebrate Earth Day!
Posted by TAMI SEAMAN on 4/22/2015Reuse, Reduce, Recycle! Let's make keep our planet safe for future generations, before it's too late. As the lyrics of a Joni Mitchell song Yellow Taxi say, "Don't it always seem to go, that you don't know what you've got till it's gone, they paved paradise and put up a parking lot." -
Spring has Sprung and Photosynthesis has begun!
Posted by TAMI SEAMAN on 3/20/2015Chemistry is fundamental to life. Photosynthesis is a process by which light energy is converted by plants into chemical energy. We can't wait to see the beautiful flowering trees and flowers of springtime! -
Today is Albert Eintstein's Birthday
Posted by TAMI SEAMAN on 3/14/2015Albert Einstein changed the scientific world. -
Pi Day is Here!
Posted by TAMI SEAMAN on 3/14/2015Pi Day is the recognition of the relationship between a circle's diameter and its circumference. The value of Pi has over 10 trillion digits past the decimal point. To make things easier to remember the equivalent of pi is often approximated at 3.14.

